Hydroxymethylfurfural
Hydroxymethylfurfural, abbreviated as HMF, is known as an intermediate compound in the Maillard reaction and can be introduced as a quality indicator in carbohydrate-containing food products such as juice, honey, milk, coffee, and dried fruits. This compound is an aldehyde type that acts as a quality indicator in many foods containing carbohydrates and amino acids or proteins. This compound is not present in fresh, untreated food products, but it is rapidly produced during thermal processes as well as during the storage of carbohydrate-rich products such as processed fruits, honey, breakfast cereals and baked bread.
Application of hydroxymethylfurfural
Hydroxymethylfurfural (HMF) is not present in fresh food, but is naturally found in small amounts in sugary foods such as honey, fruit juice, UHT milk, etc. It is formed in foods such as vinegars, jams, alcoholic products, biscuits, coffee, dried fruits, beer, bakery products and high fructose corn syrup, etc., during heat treatment such as drying or cooking. The presence of this compound can be evidence of heating or cooking food containing sugar.
One of the foods in which the amount of HMF has a significant effect on its quality is honey. Fresh natural honey can have different levels of HMF. In the hive, honey is usually below 1 mg/kg, but its amount increases with increasing ambient temperature, storage conditions and use of metal containers.
Hydroxymethylfurfural is mostly used as a quality indicator and food processing. It is used as an index to evaluate the thermal quality and preservation of food. Additionally, it is used in scientific research to investigate the effects of heat and processing on food quality, as well as as an indication of chemical changes in food over time. HMF alone has few applications, but its presence in food can indicate improper thermal processes and storage.
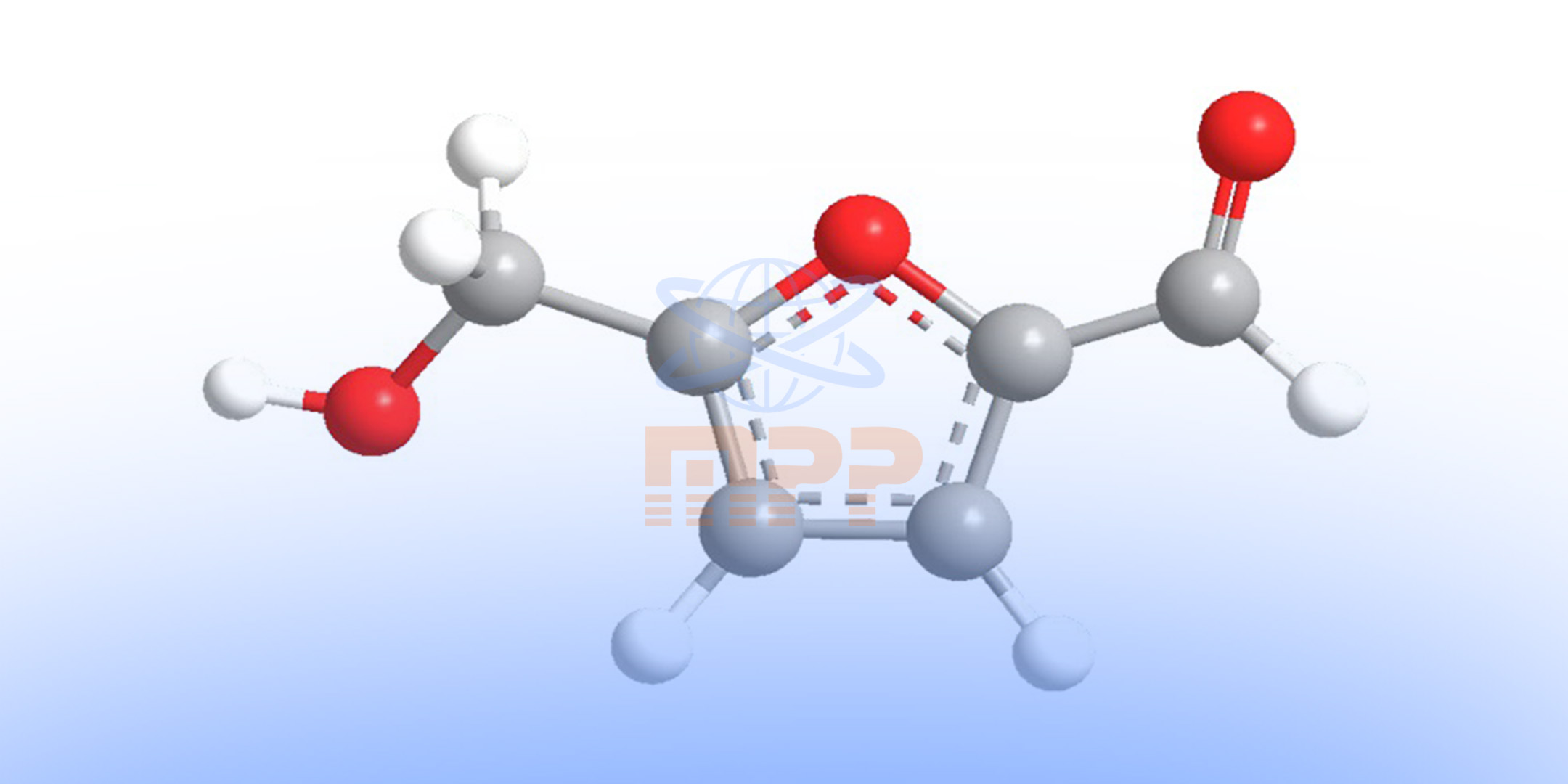
Structure of hydroxymethylfurfural
Hydroxymethylfurfural (HMF) is a cyclic aldehyde known as 5-hydroxymethylfurfural that is produced as a result of the breakdown of sugars through the Maillard reaction (a non-enzymatic browning reaction) during food processing or long-term storage. The presence of simple sugars such as glucose and fructose as well as many acids and minerals in food can increase the production of this substance.
In addition to processing, storage conditions also affect the formation of HMF, making it a suitable indicator of food quality. This compound is easily absorbed from food through the digestive system and after being metabolized into various derivatives, it is excreted through urine. HMF has harmful effects on human health, including mutagenic, genotoxic, organotoxic and enzyme inhibitors.
Furfural extract and HMF are both produced from the conversion of sugars under thermal conditions, but their production process and primary sources are different. Furfural is extracted from lignocelluloses, while HMF is mainly produced from simple sugars under heat. These two compounds can be used as quality indicators in food processing and storage, because their presence indicates chemical changes caused by heat in sugars.
Hydroxymethylfurfural in honey
Despite the healing and health-giving properties of honey, improper storage and processing can lead to the formation of undesirable nutritional compounds in this valuable product. One of these compounds is hydroxymethylfurfural (HMF), which has been considered as a carcinogenic agent in honey.
Hydroxymethylfurfural concentration is measured as the main indicator of honey temperature and heating time, which is determined by spectrophotometric methods and its maximum allowed is 40 ppm. Based on toxicological studies, the amount of HMF has been proposed as an indicator of the quality of honey and some other food products, and according to international and national standards, its maximum permissible concentration in honey has been determined to be 94 mg/kg.
Regarding the carcinogenicity of HMF, conflicting findings have been expressed by researchers, but studies have shown that the kidney, bladder and liver are more exposed to the harmful effects of HMF compared to other body tissues.
The temperature and time of the thermal process are each effective on the formation and accumulation of HMF, and with the increase in temperature and storage time, the amount of this substance increases. Considering the necessity of thermal processing to increase the quality and shelf life of honey, it is recommended that honey be heated and stored at a lower temperature in order to limit the rate of HMF formation.
The dangers of hydroxymethylfurfural
The dangers of hydroxymethyl furfural (HMF) have always been studied and debated by researchers. Some researches have shown that high consumption of this substance can have harmful effects on kidneys, eyes, bladder and liver. However, there are mixed results regarding the carcinogenicity of HMF. In general, small amounts of this compound in food are not considered a threat to human health and do not cause special concern.
Conclusion
Hydroxymethylfurfural (HMF) is an aldehyde chemical compound that is produced from the decomposition of sugars due to heat and the processing of food containing carbohydrates. This substance is used as a quality indicator in food products such as honey, juice, milk and dried fruits. HMF does not occur naturally in fresh food, but is formed during thermal processes and long-term storage. This compound can be easily absorbed by the digestive system and excreted through urine.
Although research on the dangers of HMF has had conflicting results, high consumption can have negative effects on the health of the kidneys, eyes, bladder, and liver. However, small amounts of HMF in food do not pose a serious health concern. Compliance with international standards and control of temperature and storage time can help reduce the level of HMF in food products, so it is necessary to pay attention to the amount of HMF in order to maintain the quality and safety of food.
Submit a comment
No comments found
 fa
fa
 tr
tr
 ar
ar